Students rely on Kerala Syllabus 9th Standard Chemistry Notes Pdf Download Chapter 1 Structure of Atom Extra Questions and Answers to help self-study at home.
Kerala Syllabus Std 9 Chemistry Chapter 1 Structure of Atom Extra Questions and Answers
Question 1.
Atom is electrically neutral”. Why?
Answer:
The number of electrons (negative charge) and protons (positive charge) is equal in an atom. So an atom is electrically neutral.
Question 2.
Mention the characteristics of cathode rays.
Answer:
Characteristics of cathode rays are:
- Cathode rays travel in straight lines.
- The particles of cathode rays have mass.
- Cathode rays have negative charge.
- The path of cathode rays gets deflected in the magnetic field.
Question 3.
Who was the first scientist to propose an atom model?
Answer:
J.J. Thomson was the first to propose an atom model.
Question 4.
How are electrons, positive charge and mass distributed in an atom according to the plum pudding model?
Answer:
According to the plum pudding model of the atom, positive charge and mass are uniformly distributed in the shape of a sphere. The electrons are embedded in this sphere uniformly.
Question 5.
Name the chargeless particles present in atom and mention its significance.
Answer:
Chargeless particles present in atoms are called neutrons. They have a mass equivalent to that of protons. So they, along with protons, contribute to the overall mass of the atom.
![]()
Question 6.
What is a nucleon? Where are they found in an atom?
Answer:
Protons and neutrons in an atom are called nucleons. They are found inside the nucleus of the atom.
Question 7.
What are atomic number and mass number?
Answer:
Atomic number is the total number of protons in an atom. Mass number is the total number of protons and neutrons in an atom.
Question 8.
What is maximum electrons that can be accommodated in K shell ?
Answer:
Maximum electrons K shell can accommodate is 2.
Question 9.
What are the rules for filling electrons in shells?
Answer:
Rules for electron filling in shells are
- The maximum electrons that can be accommodated in any given shell can be calculated by the equation 2n2 where ‘n’ represents the number of shells.
- Shells with lower energy will be filled with maximum number of electrons first. Thereafter shells having higher energy will get filled.
- The maximum number of electrons that can be contained in the outermost shell of an atom is 8.
Question 10.
Write down the electron configuration of the following elements. Draw their Bohr model.
Answer:
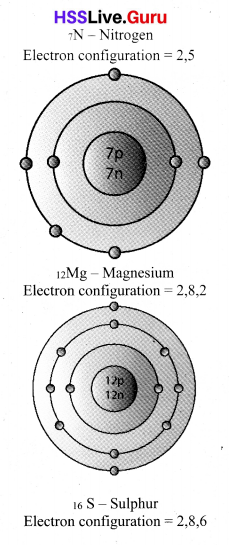
![]()
Question 11.
Fill up suitably.
Electron: J.J. Thomson
Neutron: ___________
Answer:
James Chadwick
Question 12.
Name of the scientist who suggested the planetary model of atom is ……………………… .
Answer:
Rutherford
Question 13.
Who discovered that the same negative particles are formed, when electric discharge is passed
through any gas in a discharge tube?
(Niels Bohr, James Chadwick, J.J. Thomson, Rutherford)
Answer:
J.J. Thomson
Question 14.
Choose the correct answer for the following questions from the options given in the box.
J.J. Thomson, Rutherford, Chargeless, Positive Charge, Negative Charge, Chadwick
a) Name the scientist who discovered neutron.
b) What is the charge of an electron?
Answer:
a) Chadwick
b) Negative charge
Question 15.
Match columns A, B and C suitably.
| A | B | C |
| Electron | has positive charge | not present in protium |
| Proton | Neutral | takes part in chemical reactions |
| Neutron | Has negative charge | its presence causes nuclear charge |
Answer:
| A | B | C |
| Electron | has negative charge | takes part in chemical reactions |
| Proton | Has positive charge | its presence causes nuclear charge |
| Neutron | Neutral | not present in protium |
Question 16.
Match suitably.
| Name of scientist | Name of particles | Charge of particles |
| James Chadwick | Proton | Negative charge |
| J.J. Thomson | Neutron | Positive charge |
| Rutherford | Electron | Chargeless |
Answer:
| Name of scientist | Name of particles | Charge of particles |
| James Chadwick | Neutron | Chargeless |
| J.J. Thomson | Electron | Negative charge |
| Rutherford | Proton | Positive charge |
![]()
Question 17.
Maximum number of electrons that can be accommodated in the outermost shell of an element is ______________ .
(12, 10, 8, 18)
Answer:
8
Question 18.
Which shell among the following has the highest energy?
(K, L, M, N)
Answer:
N shell has the highest energy.
Question 19.
The isotope of hydrogen used in nuclear reactors is ………………….
Answer:
Deuterium
Question 20.
Identify the isotope which is used to calculate the age of fossils and prehistoric objects.
(Deuterium, Carbon – 14, Carbon – 13, Iodine – 131)
Answer:
Carbon – 14
Question 21.
Symbols of some atoms are given, (symbols are not real)
\({ }_8^{17} \mathrm{P}\) \({ }_{18}^{40} Q\) \({ }_{8}^{16} P\) \({ }_{20}^{40} R\)
a) Which among these is an isotopic pair? Give reason.
b) How many neutrons are present in Q?
Answer:
a) \({ }_8^{17} \mathrm{P}\) and \({ }_8^{16} \mathrm{P}\) is the isotopic pair. They are the atoms of the same element with the same atomic number but different mass numbers.
b) Mass Number of element Q = 40 Atomic number of element Q = 18
No: of neutrons = Mass number-Atomic number = 40 – 18 = 22
Question 22.
Mass number of an atom is 31. There are 15 positively charged particles in its nucleus.
a) Write the electronic configuration of this atom.
b) How many neutrons are there in this atom?
Answer:
a) No: of protons (positively charged particle) = No: of electrons = 15
Electronic configuration = 2, 8, 5
b) Mass number A = 31
Atomic number Z = 15
No: ofneutrons = A – Z = 31 – 15 = 16
![]()
Question 23.
Some statements regarding atom are given below. Find the correct statements.
a) In an atom number of protons and electrons are not equal.
b) Atom is the smallest particle that can take part in a chemical reaction.
c) Atoms of different elements have same atomic number.
d) In an atom the entire mass is concentrated at its nucleus.
Answer:
d) In an atom the entire mass is concentrated at its nucleus.
Question 24.
Some elements and their electronic configuration are given in the table.
(Hint: Symbols are not real)
| Element | Electronic configuration |
| A | 2, 8, 1 |
| B | 2, 8 |
| C | 2, 8, 7 |
a) Among these elements, which has the highest stability? Give reason
Answer:
a) Element B has the highest stability due to its complete octet.
Question 25.
The isotopes of Hydrogen are given in the box.
\({ }_1^1 H\) \({ }_1^2 H\) \({ }_1^3 H\)
a) Write the name of the isotope \({ }_1^3 H\).
b) Identify the particle whose number is different in these isotopes.
Answer:
a) \({ }_1^3 H\) is known as tritium.
b) Neutrons
Question 26.
Write one use each of iodine-131 and uranium-235.
Answer:
Iodine-131 is used in the diagnosis of cancer in thyroid gland. Uranium-235 is used as fuel in nuclear reactors.
Question 27.
Atomic number of an element is 17 and its mass number is 35.
a) Find out the number of protons and neutrons in this atom.
b) Draw the Bohr model of this atom.
Answer:
a) Atomic number Z = 17
No: of proton = Atomic number = 17
Mass number of the atom A = Z + No: of neutrons = 35
No: of neutron in the atom = A – Z = 35 – 17 = 18
b) Bohr model of the atom is
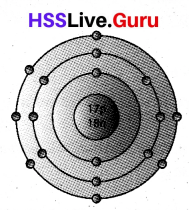
Question 28.
The mass number of an element is 27. The M shell of its atom contains 3 electrons.
a) Write the electronic configuration of this atom.
b) What is the atomic number of this element?
c) How many neutrons are present in this atom?
Answer:
a) Outermost shell (M shell) contains 3 electrons indicates that the,inner shells (i.e., K, L shells) are filled with maximum no: of electrons
i.e., electronic configuration of the atom = 2, 8, 3
b) . Atomic Number = no: of protons = no: of electrons in a neutral atom = 2 + 8 + 3 = 13
c) Mass number A = 27
No: of neutrons = Mass number – Atomic number = A – Z = 27 – 13 = 14
![]()
Question 29.
Choose the correct answer from the box and complete the table.
| Carbon – 14, Uranium – 235, Phosphorous – 31, Cobalt – 60 | |
| Use | Isotope |
| Used as fuel in atomic reactors | |
| Used to determine age of fossils | |
| Used in medical field | |
Answer:
| Use | Isotope |
| Used as fuel in atomic reactors | Uranium – 235 |
| Used to determine age of fossils | Carbon – 14 |
| Used in medical field | Cobalt – 60 |
Question 30.
Symbol of an atom is given \({ }_11^23 Na\).
a) Find the number of protons and neutrons in this atom?
b) How many shells are occupied by electrons in this atom?
Answer:
a) Mass number A = 23
Atomic number Z = 11
No: of protons = Atomic number Z = 11
No: of neutrons = Mass number-Atomic number = 23 – 11 = 12
b) NQ: of electrons = 11
Electronic configuration = 2, 8, 1
Electrons in the Na atom occupies three shells, i.e., K, L, M
Question 31.
An Aluminium atom (Al) has 13 electrons and 14 neutrons.
a) Find its mass number.
b) Draw the Bohr model of Aluminium atom.
c) Which shell of this atom has the highest energy?
Answer:
a) No: of electrons = 13
No: of protons = 13
No: of neutrons =14
Mass number = No: of protons + No: of neutrons = 13 + 14 = 27
b) Bohr model of Aluminium atom

c) M shell in aluminium atom has highest energy because as the distance from nucleus increases, energy of the shell increases.
Question 32.
The mass number of an atom is 35. The number of electrons present = 17
a) Find its atomic number.
b) How many neutrons are present in it?
c) What is its valency?
d) Write the electronic configuration of the inert gas coming just after this element.
Answer:
a) Mass number of atom A = 35
No: of electrons = 17 .
Atomic number Z = No: of electrons = 17
b) No: of neutrons = Atomic Number = 17
c) Valency is the combining power of an atom in regard to making a chemical bond with another atom or with itself.
d) Electronic of the given element = 2, 8, 7
Electronic configuration of the inert gas coming just after the given element = 2, 8, 8
Inert gases have electronic configuration with complete octet in their outermost shell.
Question 33.
The mass number of an atom X is 23. Its M shell contains 1 electron, (symbol X is not real)
a) Write the electronic configuration of X.
b) Find the total number of particles present in its nucleus.
c) How does X attain octet electronic configuration in chemical reactions?
d) Write the chemical formula of the compound formed when X reacts with oxygen.
[Hint: valency of oxygen = 2]
Answer:
a) M shell of X (outermost shell) contains 1 electrons. Therefore, the inner shells K and L contains 2 and 8 electrons respectively.
The electronic configuration of X = 2, 8, 1
b) Mass number of element X = 23
Total number of particles in nucleus is the sum of neutrons and protons (mass number) = 23
c) X attain octet electronic configuration in a chemical reaction by losing an electron, i.e., valency of X is 1
d) X2O
![]()
Question 34.
Three isotopes of carbon are C – 12, C – 13, C – 14.
[Hint: Atomic Number of C = 6]
a) Find out the number of protons in each of the above isotopes?
b) Which is the particle that differ in its number in isotopes?
c) How many neutrons are there in C – 13 isotope?
d) Which isotope of carbon is used to determine the age of fossils?
Answer:
a) No: of proton in the isotopes C – 12, C – 13, and C – 14 are same, i.e., 6
b) No: of neutrons are different for these isotopes.
c) No: of neutrons in C – 13 isotope = A – Z = 13 – 6 = 7
d) C – 14 isotope of carbon is used to determine the age of fossils.
Question 35.
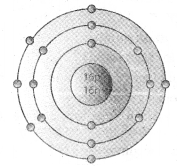
Mass number of an atom is 35. There are three shells in this atom. The outermost shell of this atom contain 7 electrons.
a) Write the electronic configuration of this atom.
b) What is its atomic number?
c) Draw the Bohr model of this atom.
Answer:
a) Outermost shell/third shell carry 7 electrons.
Therefore, the electronic configuration is 2,8,7.
b) Atomic number = No: of protons = No: of electrons = 2 + 8 + 7 = 17.
c) Bohr model of the atom is
Question 36.
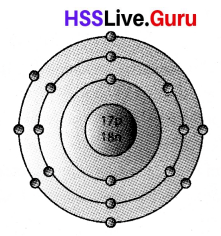
The symbol of Argon atom is \({ }_{18}^{40} \mathrm{Ar}\).
a) How many electrons are there in an Argon atom?
b) Draw the Bohr model of this atom.
c) Which shell of Argon atom has the highest energy?
Answer:
a) No: of electrons = 18
b) Bohr modle of Ar atom

c) M shell of argon atom possess highest energy.